
Summary of evaluation and recommendationsġ2.3 Other consequential or supportive therapyġ2.4 Controversial issues and areas of use where there is insufficient information to make recommendationsġ3.4 Pharmaceutical incompatibilities and drug interactionsĪtropine is the best-known member of a group of drugs known as muscarinic antagonists, which are competitive antagonists of acetylcholine at muscarinic receptors. Pharmaceutical formulation and synthesisĥ.2 Methods for identification of antidoteĥ.3 Methods for analysis of atropine in biological samplesĥ.4 Methods for analysis of the toxic agent in biological samplesġ1.1 Dose and duration of atropine sulphate therapyġ1.3 Assessment of optimal atropinizationġ1.5 Role of other anticholinergic agentsġ2.

The World Health Organization does not warrant that the informationĬontained in this publication is complete and correct and shall notīe liable for any damages incurred as a result of its use.Ĥ. Names of proprietary products are distinguished by initial capital World Health Organization in preference to others of a similar Products does not imply that they are endorsed or recommended by the The mention of specific companies or of certain manufacturers'
Atropine antidote for full#
Dotted lines on maps represent approximate border linesįor which there may not yet be full agreement. The legal status of any country, territory, city or area or of itsĪuthorities, or concerning the delimitation of its frontiers orīoundaries. Whatsoever on the part of the World Health Organization concerning This publication do not imply the expression of any opinion +41 email: designations employed and the presentation of the material in Translate WHO publications - whether for sale or for noncommercialĭistribution - should be addressed to Publications, at the above address (fax: Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 Publications of the World Health OrganizationĬan be obtained from Marketing and Dissemination, World Health National Poisons Centre, Penang, Malaysia, 18-20 December, 2002.Ģ Pharmacist-in-Charge, ACT Poisons Information Service, Canberra, Australiaģ Director, Medical Toxicology Centre, Mashad, IranĤ Deputy Director, National Institute of Occupational Health, Ahmedabad, Indiaĥ Chinese Centre for Disease Control and Prevention, Beijing, ChinaĪll rights reserved. Robin McKeown PhC 2 August 2002, Geneva, Switzerland ORGANOPHOSPHORUS PESTICIDES Monograph on Atropine INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY EVALUATION Injectables: 1 mg/mL, 0.5 mg/mL, 15mg/mL, 0.Atropine (International Programme on Chemical Safety Evaluation, 2002) When urine from the animal suspected of atropine poisoning is instilled in the eye of a cat, dilatation can be noticed.Īntidote of Atropine is Physostigmine Preparations Signs of Atropine toxicity includes dry mouth, thirst, constipation, mydriasis, tachycardia, restlessness, delirium, ataxia, convulsions, respiratory depression and respiratory failure leads to death. Herbivores are more resistant than carnivores, Certain strains of rabbits are resistant to a diet of belladonna leaves, (as the liver contains the enzyme atropinase) although eating their meat may be toxic if eaten by dogs, cats or humans.
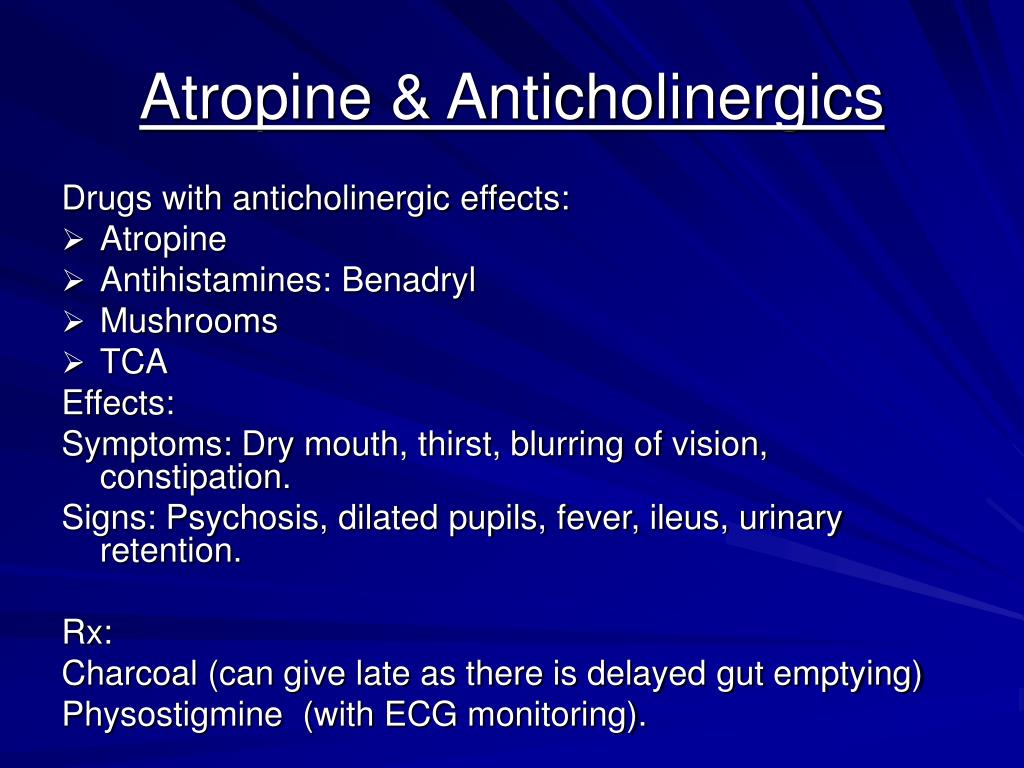

Atropine is a natural Anticholinergic (Parasympatholytic) and Muscarinic receptor antagonists agent that is present in Atropa belladonna leaves and in some other plants of the Solanaceae family.


 0 kommentar(er)
0 kommentar(er)
