
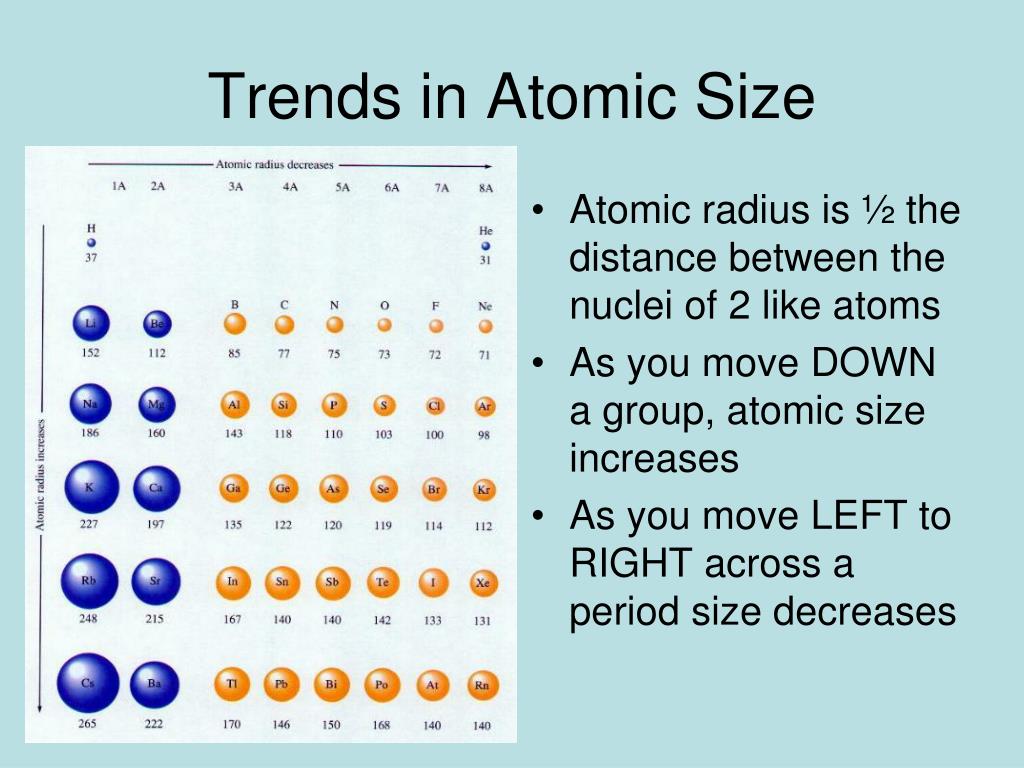
The internal energy levels “shield” and reduce electrostatic attraction of the valence electrons to the protons. (2) Number of energy levels: The greater the number of energy levels, the larger the atomic radii. There are three main factors that affect the size of the atoms: the nuclear charge of the atom, the shielding effect, and the number of energy levels that. Group I elements have low ionization energies because the loss of an electron forms a stable octet.
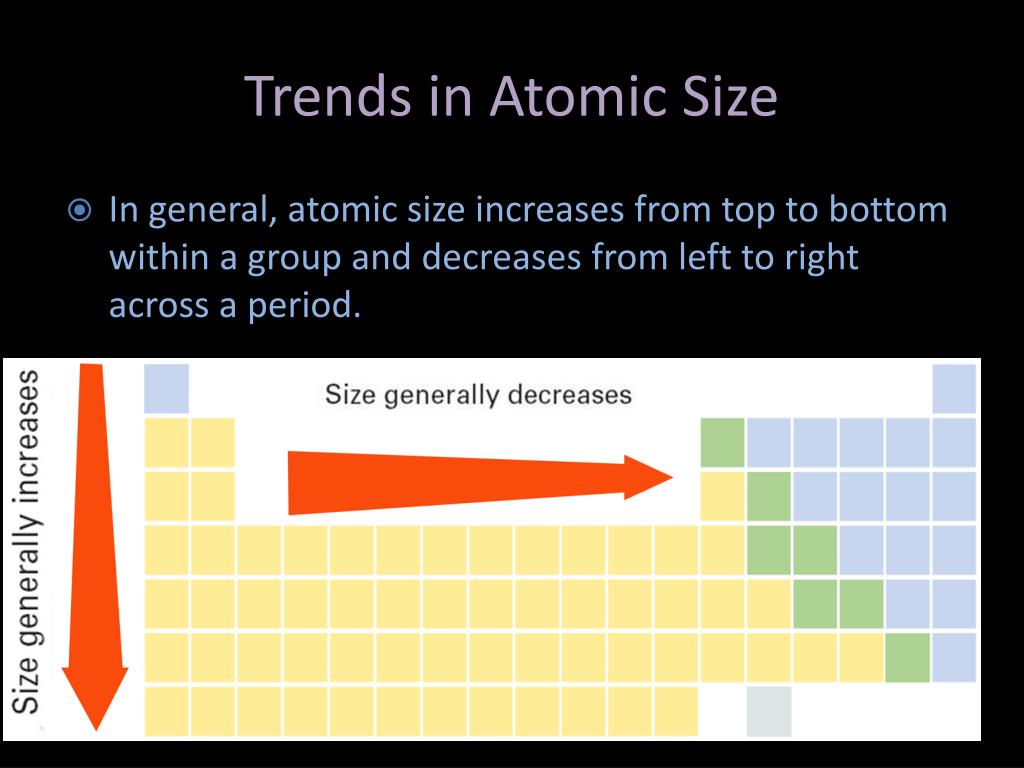
(1) Nuclear charge (number of protons) : The stronger the ‘pull’ the protons have to the electrons with electrostatic attraction, then the smaller the size of the atom radii Ionization energy decreases moving down a group (increasing atomic radius). ii Size of the atom: With the increase in size of the atom the distance. This is because as you go down a group, the number of. This is due to greater attraction for the incoming electron if nuclear charge is high. Atomic Radii is affected by two main factors : The atomic radius trend is that as you go down a group on the periodic table, the atomic radius decreases.


 0 kommentar(er)
0 kommentar(er)
